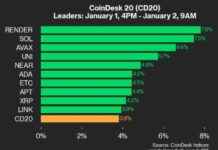
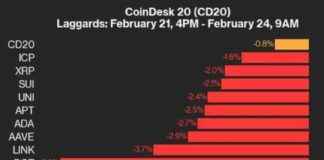
Ilya Lichtenstein, the husband of rapper Heather Morgan, also known as “Razzlekhan,” has been sentenced to five years in prison for his involvement in the theft of around 120,000 bitcoin from Bitfinex in 2016. The U.S. Department of Justice announced the sentencing on Thursday, highlighting how Lichtenstein used advanced hacking tools and techniques to hack into the network.
Once inside Bitfinex’s network, Lichtenstein fraudulently authorized over 2,000 transactions that transferred 119,754 bitcoin to his own wallet. To cover his tracks, he deleted access credentials and log files that could have exposed his actions to law enforcement.
Following the theft, Lichtenstein and his wife engaged in laundering the stolen funds. They managed to launder 25,111 bitcoin, which accounted for 21% of the total stolen amount, by using Eastern European bank accounts and bitcoin mixing services. Prosecutors described their laundering methods as the most complicated techniques they had seen, involving automation of transactions, depositing funds into darknet markets and exchanges, converting bitcoin to other cryptocurrencies, using mixing services, and exchanging funds for gold coins.
Despite the complexity of their methods, former cybercrime leader Brett Johnson criticized Lichtenstein’s approach, pointing out flaws like using Coinbase accounts directly linked to him. Lichtenstein and Morgan initially faced suspicions of money laundering until Lichtenstein admitted to being the hacker. They pleaded guilty to one count of conspiracy to commit money laundering, a charge that carries a maximum sentence of 20 years in prison.
In addition to the five-year prison sentence, Lichtenstein will serve three years of supervised release. Heather Morgan is set to be sentenced on November 18, with prosecutors recommending an 18-month sentence for her involvement in the money laundering scheme.
It’s essential to note that CoinDesk, the media outlet covering this story, upholds strict editorial policies to ensure integrity, editorial independence, and freedom from bias in its publications. CoinDesk is part of the Bullish group, which invests in digital asset businesses, and employees, including journalists, may receive equity-based compensation from the group. The case of Ilya Lichtenstein and Heather Morgan sheds light on the intricate world of cryptocurrency theft and money laundering, showcasing the challenges authorities face in combating such crimes.